Bringing a fiberglass cast bandage to market requires a robust quality system and clear technical evidence to ensure patient safety, clinical performance, and regulatory compliance—especially for export to the EU. This overview maps the core ISO frameworks and CE requirements from QMS and risk management to labeling, performance evidence, and post-market surveillance.

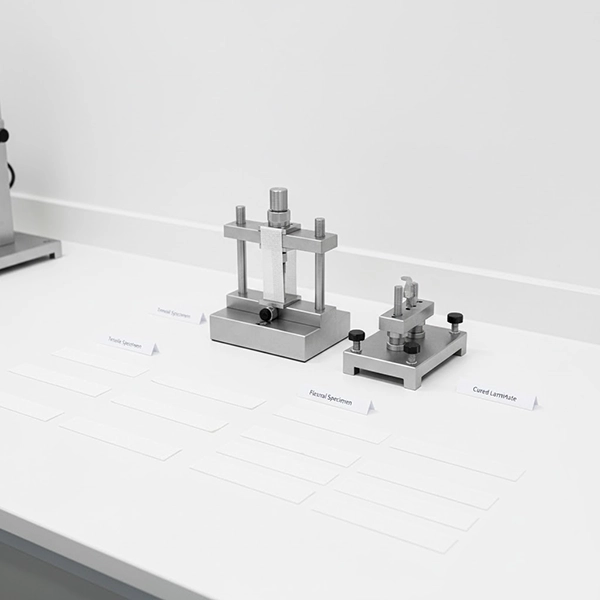

ISO/CE alignment is not just a regulatory checkbox—it’s a framework for consistent quality and market trust. Explore our export-ready fiberglass casting tapes on the product page.